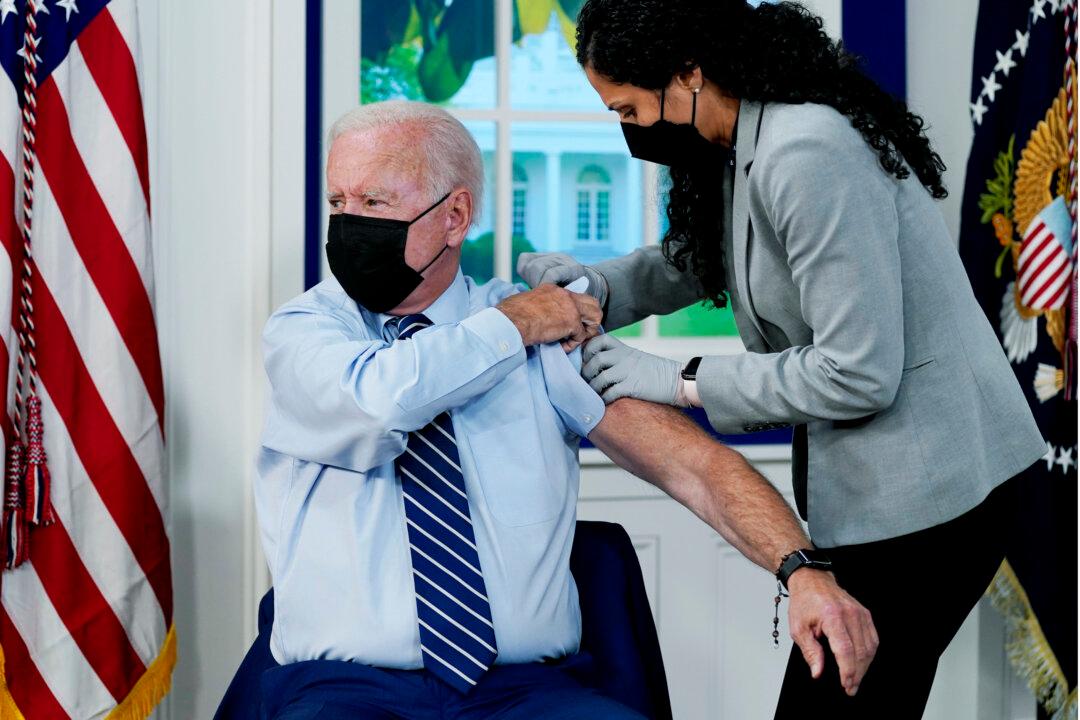
President Joe Biden received his COVID-19 vaccine booster shot on camera in the White House Monday just days after a third dose was recommended for certain groups by a CDC advisory committee.
Third doses of the Pfizer-BioNTech vaccine have been approved by the FDA and CDC for people over the age of 65, people over the age of 18 with underlying health conditions, and those who are at high risk of catching the virus because of their occupation.