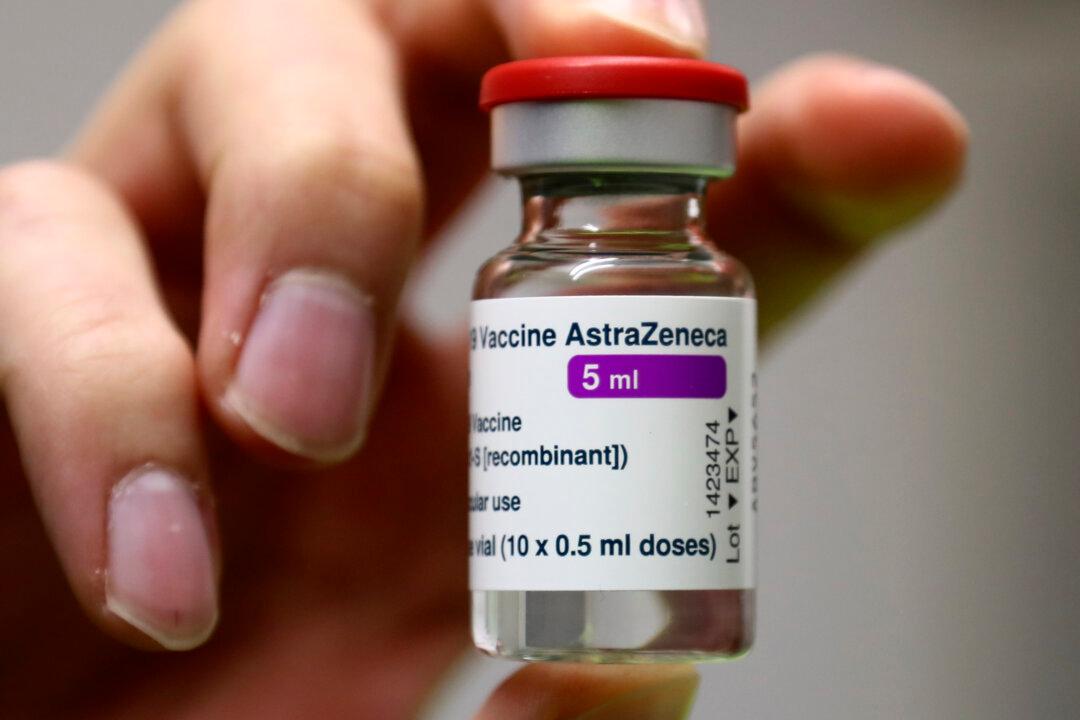
Results from a U.S. clinical trial testing the efficacy and safety of AstraZeneca’s COVID-19 vaccine may have included “outdated information,” American officials said early Tuesday.
The Data Safety Monitoring Board notified the company and two health agencies late Monday that it was worried by the information AstraZeneca released on its trial.