The World Health Organization (WHO) has been authorized to use the monkeypox (mpox) vaccine on adults.
The WHO said on Friday that the MVA-BN vaccine—the first against mpox—has been added to its prequalification list.
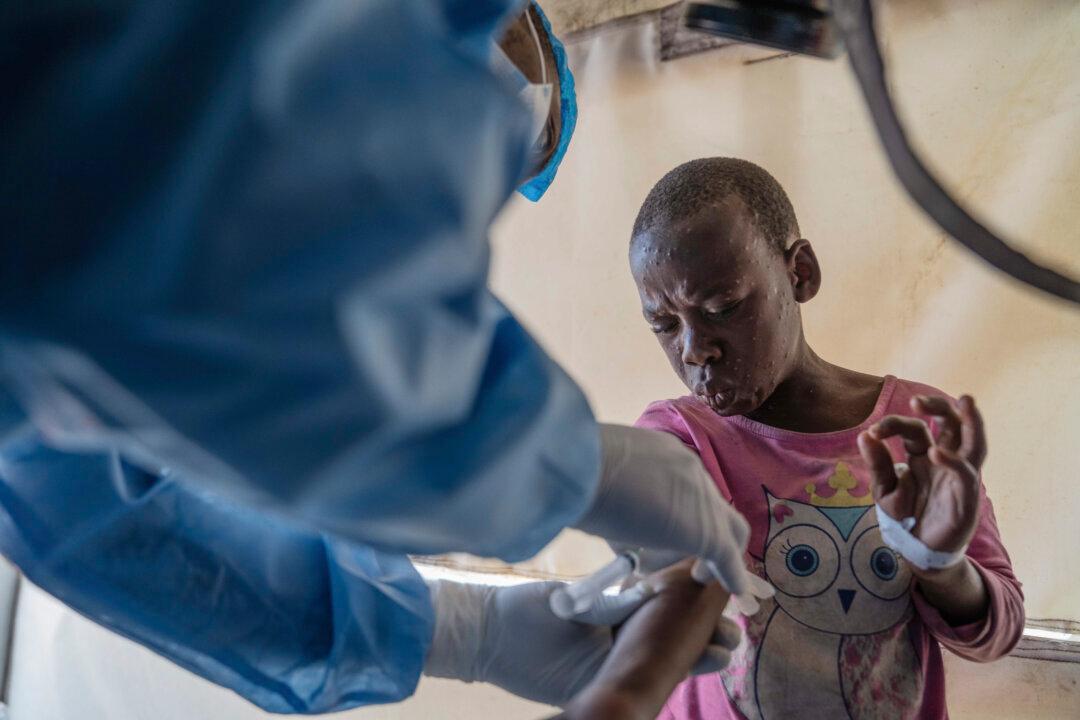
The World Health Organization (WHO) has been authorized to use the monkeypox (mpox) vaccine on adults.