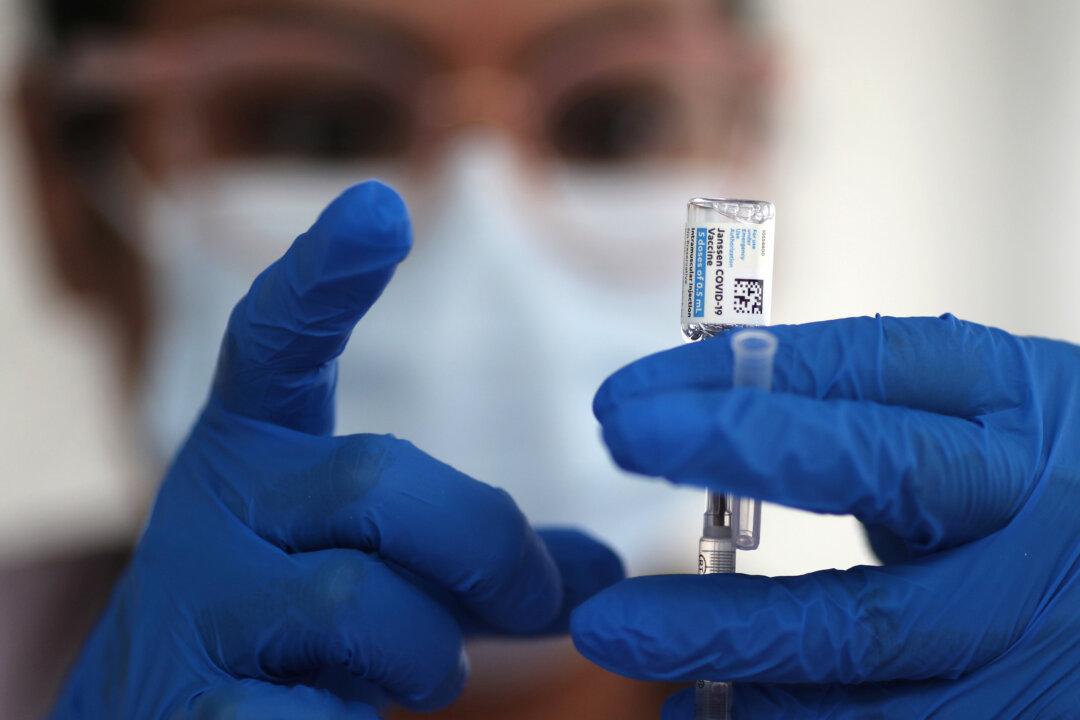
The White House and top health officials indicated Tuesday that a pause on the Johnson & Johnson single-shot COVID-19 vaccine won’t have a lasting or significant impact on the federal government’s vaccination plan.
Earlier in the day, the Food and Drug Administration (FDA) and Centers for Disease Control (CDC) recommended a pause on the vaccines after reports of rare blood clots among recipients. The agencies are investigating blood clots in six women days after they got the shot.