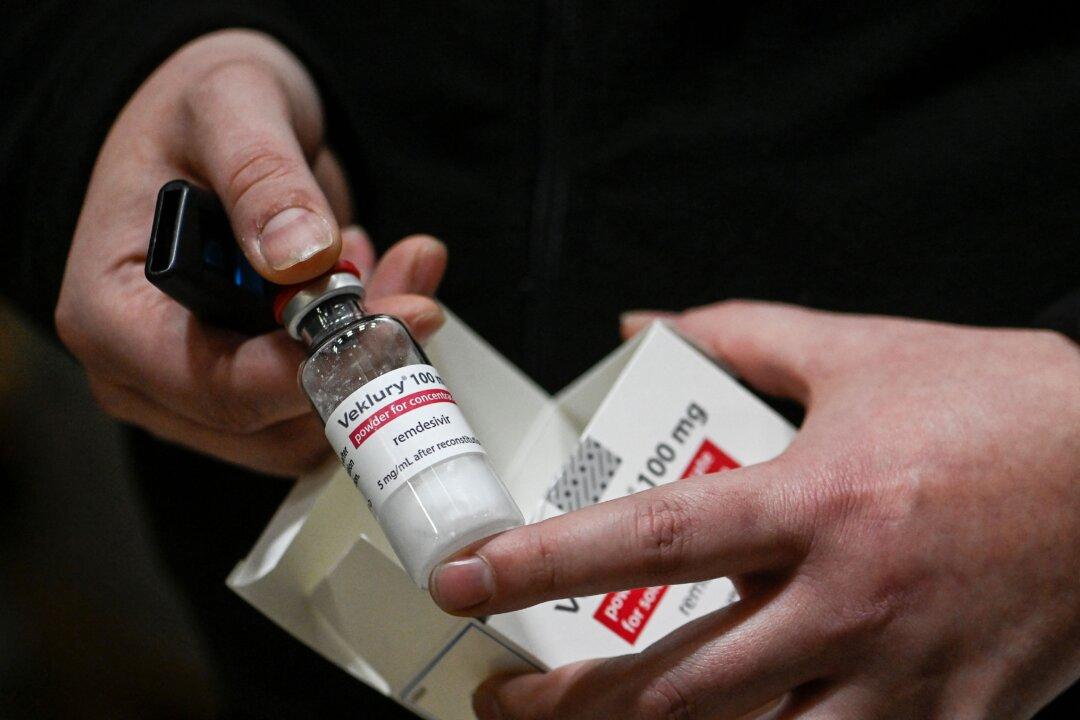
Some nonhospitalized patients seeking treatment for COVID-19 can now use the antiviral remdesivir after U.S. drug regulators on Jan. 21 expanded approval of the drug.
The Food and Drug Administration (FDA) said the expansion was supported by a study run by Gilead Sciences, which developed remdesivir, showing the drug cut the risk of hospitalization by 87 percent among people who tested positive for COVID-19.