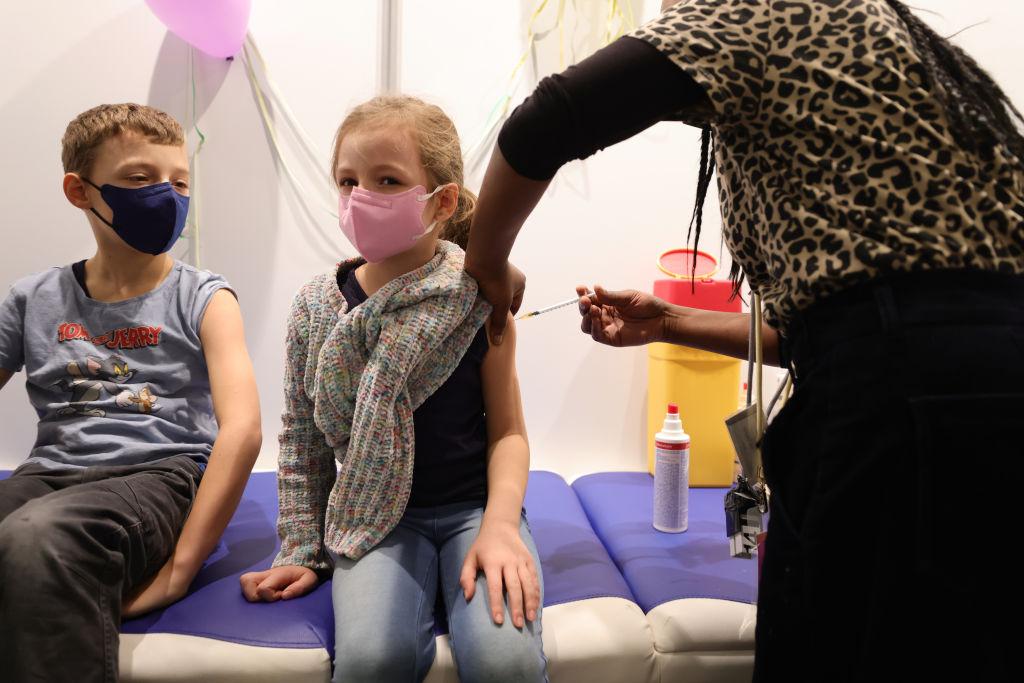The UK government’s advisory body on vaccines has recommended giving low-dose Pfizer-BioNTech COVID-19 vaccine shots to vulnerable children as young as 5, and booster shots for some teenagers.
In a statement published on Wednesday, the Joint Committee on Vaccination and Immunisation (JCVI) said two 10 microgram doses of the Pfizer vaccine, also known as Comirnaty, should be offered to children aged 5 to 11 who are either in a clinical risk group or live with an immunosuppressed person.