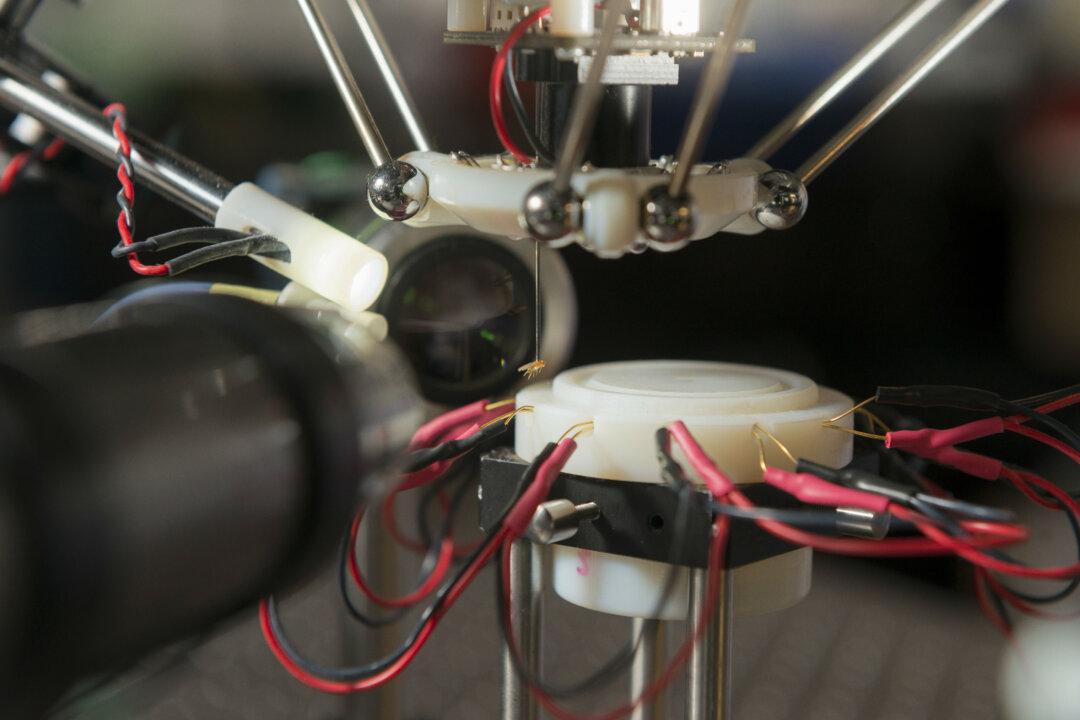
Two new tools are letting scientists see brain activity as it happens live.
The probes involve proteins that light up as an electric current sweeps down the long tendrils that link nerves together. The scientists can insert these proteins into a specific group of brain cells that they want to study—say, cells in the part of the brain involved in memory, or cells that specifically inhibit other neurons from firing—and then watch those cells as they communicate.
“You want to know which neurons are firing, how they link together, and how they represent information,” says Michael Lin, assistant professor of pediatrics and of bioengineering at Stanford University. “A good probe to do that has been on the wish list for decades.”
Lin and Mark Schnitzer, associate professor of biology and of applied physics at Stanford, developed two different approaches to allow neuroscientists to read brain activity more quickly and sensitively. Their research papers on this topic were published in Nature Neuroscience (Lin’s study) and Nature Communications (Schnitzer’s study).
Develop Drugs, Study Disease
With these tools scientists can study how we learn, remember, navigate, or any other activity that requires networks of nerves working together. The tools can also help scientists understand what happens when those processes don’t work properly, as in Alzheimer’s or Parkinson’s diseases, or other disorders of the brain.
The proteins could also be inserted in neurons in a lab dish. Scientists developing drugs, for example, could expose human nerves in a dish to a drug and watch in real time to see if the drug changes the way the nerve fires. If those neurons in the dish represent a disease, like Parkinson’s disease, a scientist could look for drugs that cause those cells to fire more normally.
For more than a decade, neuroscientists have watched a proxy of nerves firing. Each time a nerve sends a signal, calcium floods into the cell and is then pumped back out in anticipation of the next signal.
Watching the Shadows
In fact, Schnitzer developed a miniature camera that he has been using to peer into the brains of mice to record these calcium waves. His lab has focused on studying the region of the brain involved in learning and memory.
But what Schnitzer sees through his tiny camera isn’t the actual nerve activity. He has been watching the shadows, and like any shadows they are a good proxy—but their shapes aren’t always realistic.
Calcium stays in the neuron long after a signal has swept past, and may mask a second signal as it flashes by. Also, sometimes an electrical signal won’t trigger enough calcium to enter a cell for the protein to light up.
“Sensing calcium is insufficient for a full understanding of what’s happening,” Schnitzer says. “There are also many neuronal cell types that are not well studied with calcium probes.”
Frustrated with the state of effective tools for watching nerves fire, Lin and Schnitzer applied for and received a seed grant from Stanford Bio-X to develop one. These grants support high-risk projects that bring together engineering and biology know-how to solve problems in the field.
The First Approach
Although the two labs had the same goal and ended up developing probes with similar qualities, they took very different approaches.
Lin’s lab focuses on engineering proteins that can be used as tools to study aspects of how the cell functions. Lin and a postdoctoral fellow in his lab, Francois St-Pierre, had an idea for generating a protein that would light up in response to a change in voltage, such as what happens when a nerve sends a signal.
Other scientists were working on the same problem, but they were not able to create a protein that responded quickly and strongly to a change in voltage. By looking at the structure of different voltage-sensing proteins, St-Pierre thought he could generate a better signal by putting the fluorescent element in the middle of a voltage-sensing protein.
Despite some concerns that a big fluorescent element in the middle of the protein might disrupt its function, the combination worked. He and Lin named their probe ASAP—an acronym for a scientific description of the protein as well as a description of the protein’s speedy light. St-Pierre was first author on the Nature Neuroscience paper.
A Second Approach
Like St-Pierre, postdoctoral scholar Yiyang Gong in Schnitzer’s lab recognized the need for a voltage sensing protein, but he took inspiration from a different approach. He had read about work by scientists attempting to detect voltage starting with bacterial proteins called rhodopsins—but without much success.
Gong made significant modifications to that approach and, like St-Pierre, ended up with a protein that will embed in the nerve cell membrane and produce light when the nerve fires. Gong was first author on the Nature Communications paper.
“The two probes actually have similar performance, which is a coincidence because we arrived at them from very different directions,” Lin says.
Tested in Living Mice
Both groups show that their proteins work in neurons in a lab dish. Gong also inserted his protein in a group of neurons (called Purkinje neurons) in living mice and was able to record the protein’s flashing light as those nerves sent signals.
He was able to see those nerves fire through a tiny glass window into the mouse brain, but the scientists say they could use a camera like the one Schnitzer developed to observe deeper parts of the brain.
The scientists say they view their probes as a starting point. They expect to continue refining the proteins to have properties that are optimized for different cell types or to produce different colors of light.
“I think there will be exciting applications enabled by what we have developed,” Schnitzer says.
Source: Stanford University. Republished from Futurity.org under Creative Commons License 3.0.
*Image of “nerve cell“ via Shutterstock