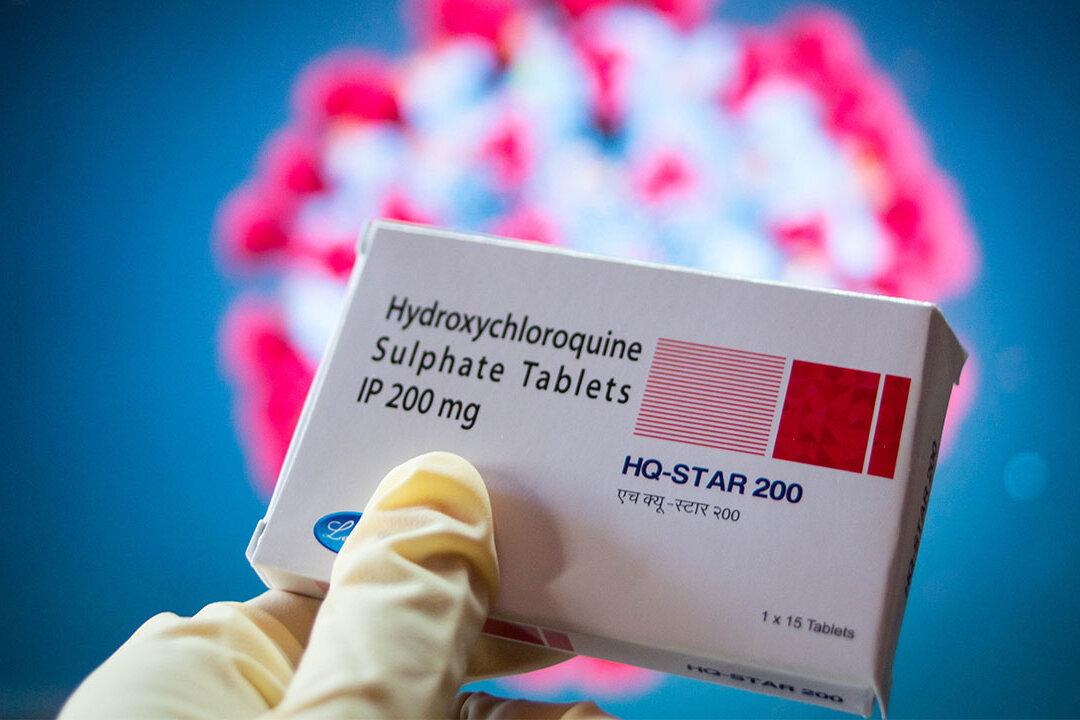
An investigation has found that among the hundreds of COVID-19 research papers that have been withdrawn, a retracted study linking the drug hydroxychloroquine to increased mortality was the most cited paper.
With 1,360 citations at the time of data extraction, researchers in the field were still referring to the paper “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis” long after it was retracted.