Stem Cell Treatment Trialed on Human for First Time in US
Stem cell treatments were administered to the first US citizen, a sufferer of a spinal cord injury.
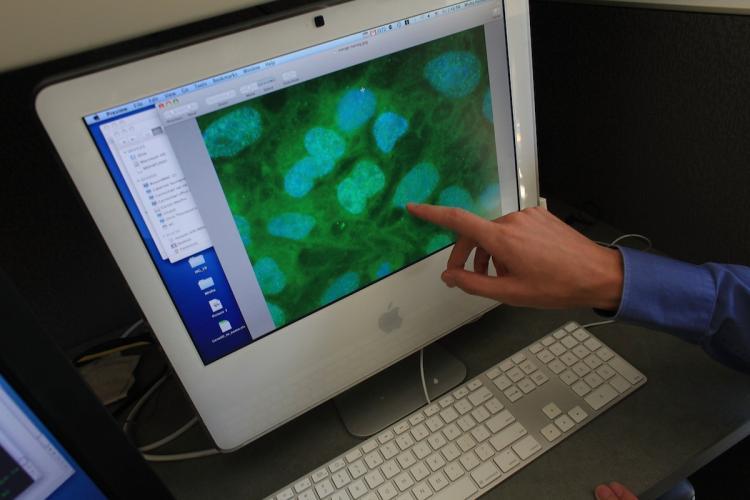
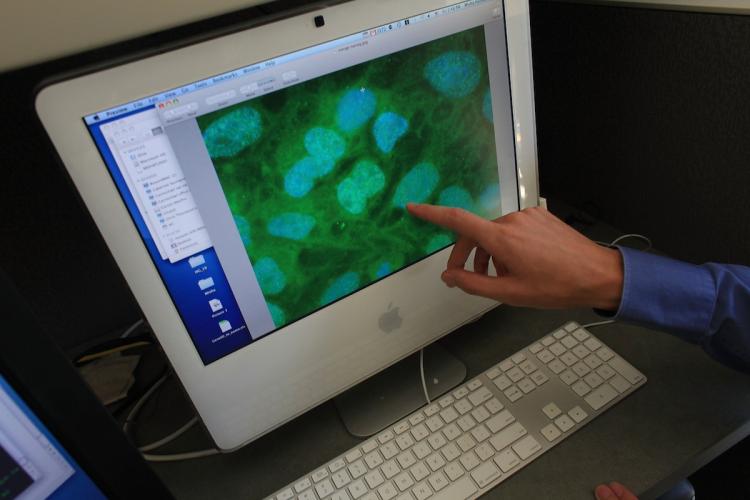
Stem cells are viewed on a computer screen at the University of Connecticut's (UConn) Stem Cell Institute in Farmington, Connecticut. For the first time in the US, a person with a spinal cord injury is being treated with injections with the controversial process of human embryonic stem cells. Spencer Platt/Getty Images
|Updated:
Mary Silver writes columns, grows herbs, hikes, and admires the sky. She likes critters, and thinks the best part of being a journalist is learning new stuff all the time. She has a Masters from Emory University, serves on the board of the Georgia chapter of the Society of Professional Journalists, and belongs to the Association of Health Care Journalists.
Author’s Selected Articles