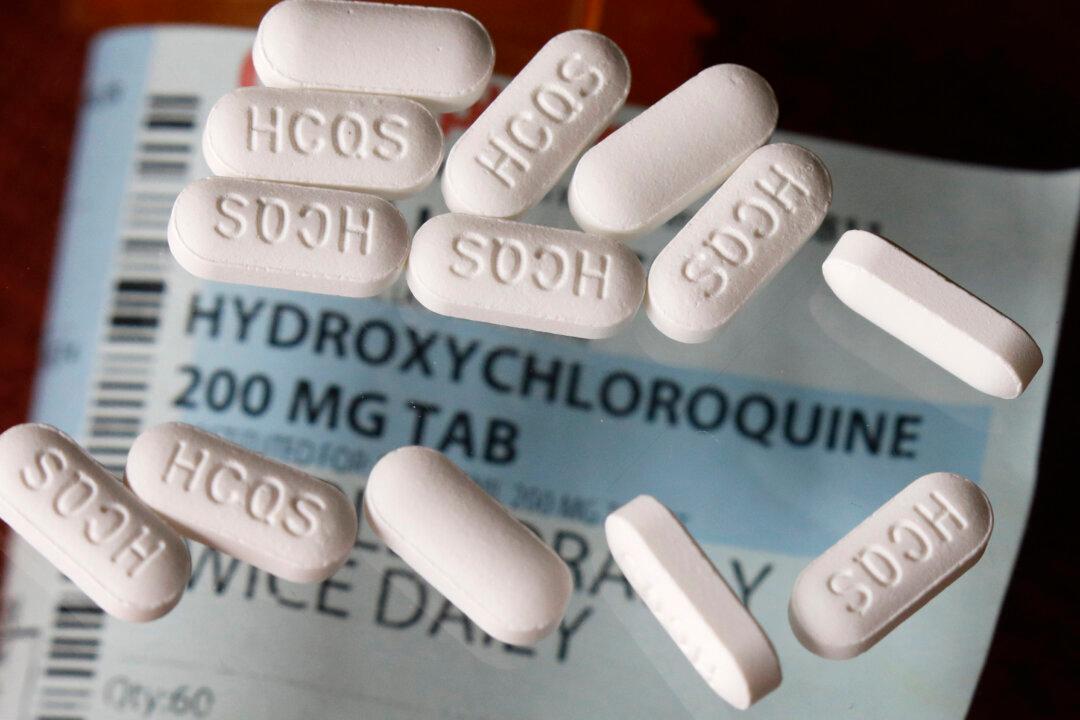
Swiss drugmaker Novartis gained permission from the U.S. Food and Drug Administration to test hydroxychloroquine, a malaria drug being widely used against COVID-19, against the new disease from China.
COVID-19 is caused by the CCP (Chinese Communist Party) virus, a novel coronavirus.