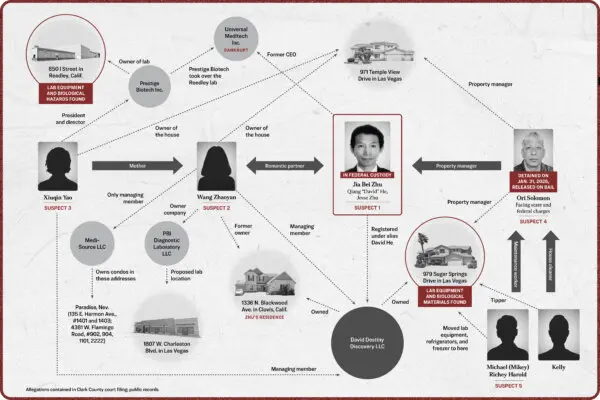
COVID-19 vaccines cause heart inflammation, U.S. authorities now acknowledge. But after being warned in early 2021 about a “large number” of cases among healthy, young people in Israel after COVID-19 vaccination, authorities did not immediately alert the public while also failing to detect a safety signal that was present in the United States, an Epoch Times investigation has found.
Even after deaths from myocarditis—inflammation of the heart—were reported and myocarditis was designated as a likely side effect of the shots, U.S. officials kept recommending vaccination for virtually the entire populace.