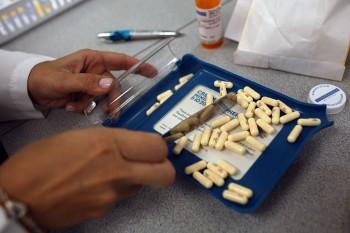
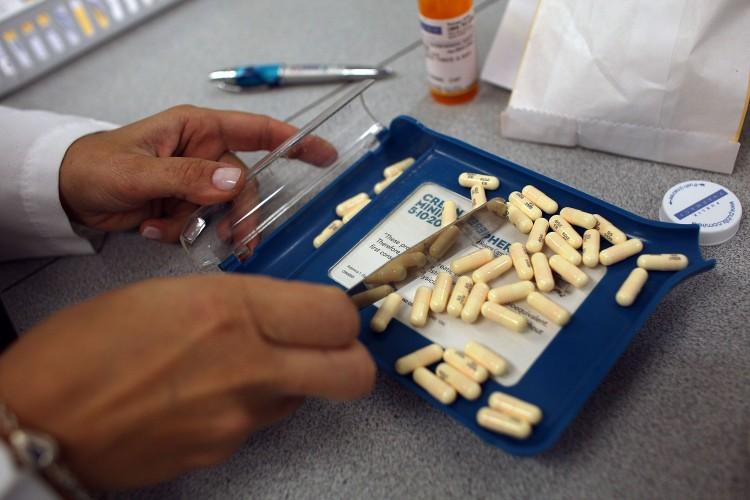
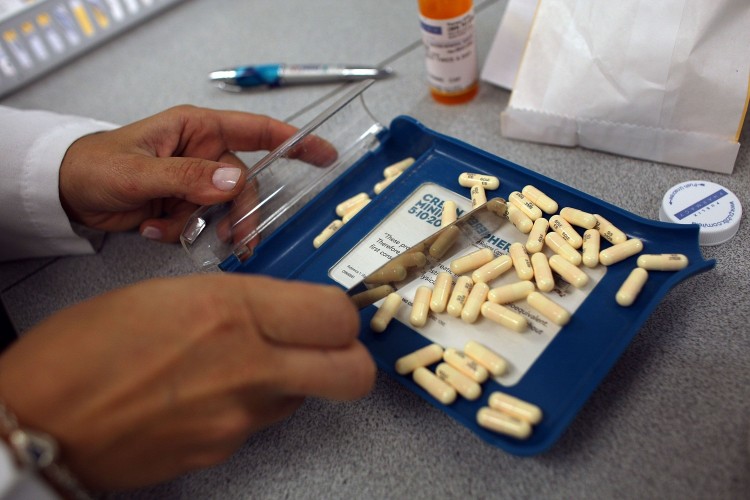
Through a series of talks in several provinces, an Ontario MP is continuing his push for an independent agency to oversee the approval of pharmaceutical drugs in Canada.
Conservative MP Terence Young, author of “Death by Prescription,” returned late last week from his latest cross-Canada tour to expose what he calls “ongoing corporate malfeasance” that causes more than 200,000 deaths per year in North America.
“It’s to raise awareness on issues around prescription drug safety and to help empower patients to keep themselves and their families safe,” Young says.
“Prescription drugs taken as prescribed—in other words, the right way—are the fourth leading cause of death in North America today.”
For over a decade, Young has been leading a charge to increase oversight, eliminate conflicts of interest from regulatory agencies, and require more testing for drugs approved for the market and the health care system.
He is calling for an independent agency that would have the power to order—not negotiate—unsafe drugs off the market, and the power to prepare and distribute clearly worded warnings for doctors and patients for all approved drugs.
Young has been fighting for a more stringent drug-monitoring system since of the death of his daughter in 2000. Fifteen-year-old Vanessa Young died suddenly of suspected complications from the Johnson & Johnson drug Prepulsid, prescribed for bloating.
Prepulsid was pulled from the market soon after, but not before becoming a “blockbuster” drug with $1 billion in sales over eight years. At the time, a reported 80 deaths had been linked to the drug worldwide.
Young has since become a strong critic of the pharmaceutical industry, and founded Drug Safety Canada to advocate for safe prescription drugs.
The MP writes in his book of the “totally inadequate warnings that patients get from doctors and Health Canada,” noting that the list patients receive categorizes only the most common side effects of a drug and often neglect to mention more dangerous side effects.
He says the relationship between drug companies and Health Canada regulators has been problematic since budget cuts in the 1990s forced the department to depend on drug company user fees for a large part of their funding.
“Health Canada back in 1997 was encouraged to ‘partner’ with the pharmaceutical industry. At that time they closed the government labs that tested drugs. They encouraged Health Canada to partner with the industry and to help them to market drugs, which is utterly inappropriate,” Young says.
“You don’t tell restaurant inspectors it’s their job to help restaurants sell food. You don’t tell air traffic controllers to hurry up and get those planes in faster, which is what happens with drugs in the way that they get reviewed and approved.
“Right now, above 60 percent of the costs for drug regulation are paid for by the pharmaceutical industry.”
Another concern Young raises is Health Canada’s lack of transparency in how it approves drugs. Comparisons have been drawn to the U.S. Food and Drug Administration’s policy of having full review reports published online after drugs are approved.
The USFDA also demands that companies submit data from clinical drug trials. The agency conducts its own verification of the results and also catalogues and posts the results. All aspects of drug approvals by the USFDA are subject to public input.
In contrast, very little of the drug approval process is made public in Canada.
Health Canada maintains that legal restrictions limit what it can reveal publicly about drugs and medical device submissions.
“The confidentiality of such submissions is protected by Canadian common law, several federal statutes, and international trade obligations,” Leslie Meerburg, a Health Canada spokeswoman, told the Toronto Star in March.
“Canada is also a signatory to several international instruments that guide what information may and may not be disclosed.”
Meerburg also said the agency has made information available on its website regarding the quality and safety of a drug submission, including summaries of clinical trials and pre-market adverse events.
The Therapeutic Products Directorate, the agency in charge of approving pharmaceuticals for the market, consists of medical health professionals, according to Health Canada.
Since 1997, 27 drugs approved by Health Canada as safe were taken off the market for harming or killing patients, Young says.
Conservative MP Terence Young, author of “Death by Prescription,” returned late last week from his latest cross-Canada tour to expose what he calls “ongoing corporate malfeasance” that causes more than 200,000 deaths per year in North America.
“It’s to raise awareness on issues around prescription drug safety and to help empower patients to keep themselves and their families safe,” Young says.
“Prescription drugs taken as prescribed—in other words, the right way—are the fourth leading cause of death in North America today.”
For over a decade, Young has been leading a charge to increase oversight, eliminate conflicts of interest from regulatory agencies, and require more testing for drugs approved for the market and the health care system.
He is calling for an independent agency that would have the power to order—not negotiate—unsafe drugs off the market, and the power to prepare and distribute clearly worded warnings for doctors and patients for all approved drugs.
Young has been fighting for a more stringent drug-monitoring system since of the death of his daughter in 2000. Fifteen-year-old Vanessa Young died suddenly of suspected complications from the Johnson & Johnson drug Prepulsid, prescribed for bloating.
Prepulsid was pulled from the market soon after, but not before becoming a “blockbuster” drug with $1 billion in sales over eight years. At the time, a reported 80 deaths had been linked to the drug worldwide.
Young has since become a strong critic of the pharmaceutical industry, and founded Drug Safety Canada to advocate for safe prescription drugs.
Inappropriate Relationship
The MP writes in his book of the “totally inadequate warnings that patients get from doctors and Health Canada,” noting that the list patients receive categorizes only the most common side effects of a drug and often neglect to mention more dangerous side effects.
He says the relationship between drug companies and Health Canada regulators has been problematic since budget cuts in the 1990s forced the department to depend on drug company user fees for a large part of their funding.
“Health Canada back in 1997 was encouraged to ‘partner’ with the pharmaceutical industry. At that time they closed the government labs that tested drugs. They encouraged Health Canada to partner with the industry and to help them to market drugs, which is utterly inappropriate,” Young says.
“You don’t tell restaurant inspectors it’s their job to help restaurants sell food. You don’t tell air traffic controllers to hurry up and get those planes in faster, which is what happens with drugs in the way that they get reviewed and approved.
“Right now, above 60 percent of the costs for drug regulation are paid for by the pharmaceutical industry.”
Another concern Young raises is Health Canada’s lack of transparency in how it approves drugs. Comparisons have been drawn to the U.S. Food and Drug Administration’s policy of having full review reports published online after drugs are approved.
The USFDA also demands that companies submit data from clinical drug trials. The agency conducts its own verification of the results and also catalogues and posts the results. All aspects of drug approvals by the USFDA are subject to public input.
In contrast, very little of the drug approval process is made public in Canada.
Legal Restrictions
Health Canada maintains that legal restrictions limit what it can reveal publicly about drugs and medical device submissions.
“The confidentiality of such submissions is protected by Canadian common law, several federal statutes, and international trade obligations,” Leslie Meerburg, a Health Canada spokeswoman, told the Toronto Star in March.
“Canada is also a signatory to several international instruments that guide what information may and may not be disclosed.”
Meerburg also said the agency has made information available on its website regarding the quality and safety of a drug submission, including summaries of clinical trials and pre-market adverse events.
The Therapeutic Products Directorate, the agency in charge of approving pharmaceuticals for the market, consists of medical health professionals, according to Health Canada.
Since 1997, 27 drugs approved by Health Canada as safe were taken off the market for harming or killing patients, Young says.