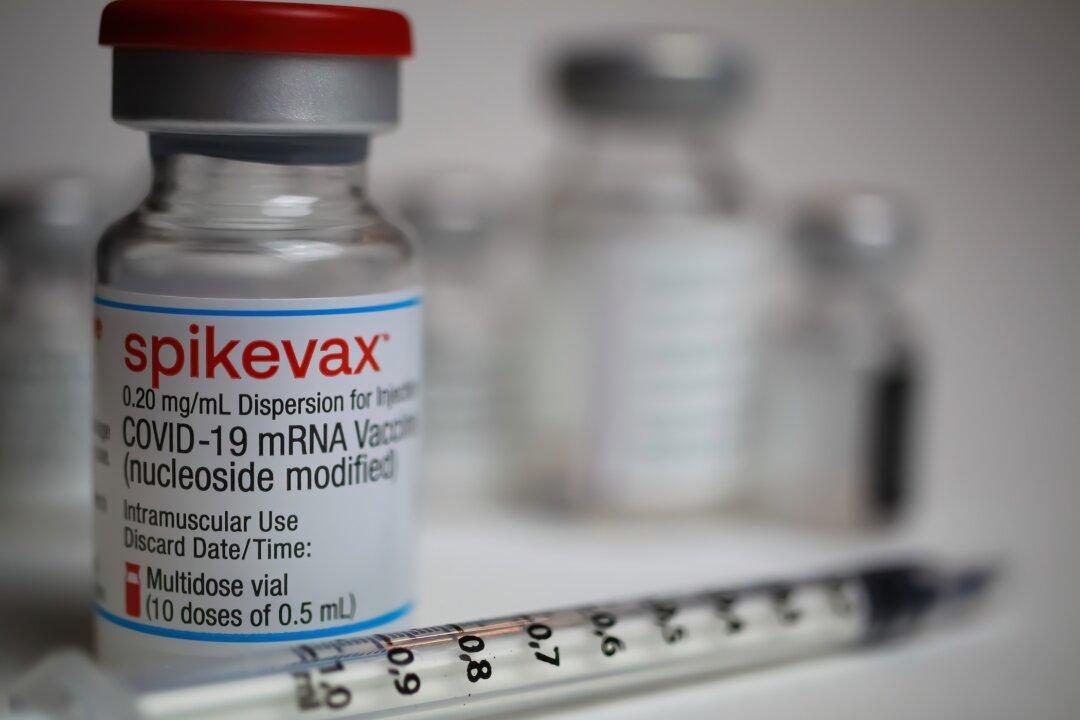
Moderna’s internal documents regarding their COVID vaccine trials, obtained via a Freedom of Information Act request by Judicial Watch, show that most of their studies submitted for approval to the FDA were “irrelevant” and did not follow Good Laboratory Practices (GLP), according to a former pharma executive.
The 700 pages contain a portion of the formal Biologics Licensing Application (BLA) package that a manufacturer is required to submit to the FDA for approval.