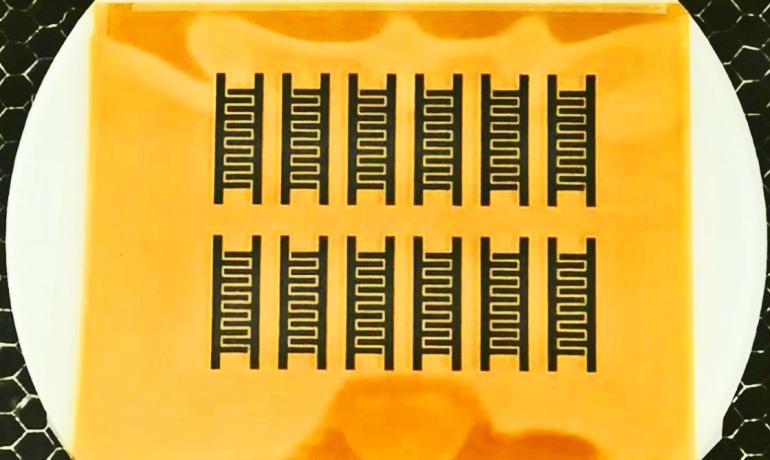
Scientists have created sheets of flexible graphene by burning a cheap polymer with a laser.
The result is a jumble of interconnected graphene flakes with five-, six-, and seven-atom rings.
The five- and seven-atom rings would normally be considered defects, but, in this case, they’re features. The process makes graphene that may be suitable for electronics or energy storage.
“This will be good for items people can relate to: clothing and wearable electronics like smartwatches that configure to your smartphone,” says James Tour, a chemistry professor at Rice University.
MORE:
- Ultra-Futuristic Smartwatch Design: Holographic Keyboard, Graphene Body
- Graphene Hybrid Foam Bounces Back
- Scientists Find Fatal Flaw in Brittle Graphene
The process works in air at room temperature and eliminates the need for hot furnaces and controlled environments.
This approach to making graphene is quite different from previous works by Tour’s lab, which pioneered the small-scale manufacture of the atom-thick material from common carbon sources, even Girl Scout cookies, and learned to split multiwalled nanotubes into useful graphene nanoribbons.
But as in the previous work, the base material for what the researchers call laser-induced graphene (LIG) is inexpensive.
“You buy polyimide flexible plastic sheets in huge rolls, called Kapton, and the process is done entirely in air with a rapid writing process. That sets it up for a very scalable, industrial process,” Tour explains.
Pretty Good Conductor
The product is not a two-dimensional slice of graphene but a porous foam of interconnected flakes about 20 microns thick. The laser doesn’t cut all the way through, so the foam remains attached to a manageable, insulating, flexible plastic base.
The process only works with a particular polymer. The researchers led by Jian Lin, a former postdoctoral research in the Tour Group and now an assistant professor at the University of Missouri, tried 15 different polymers and found only two could be converted to LIG. Of those, polyimide was clearly the best.
Tour says the resulting graphene isn’t as conductive as copper, but it doesn’t need to be. “It’s conductive enough for many applications,” he says.