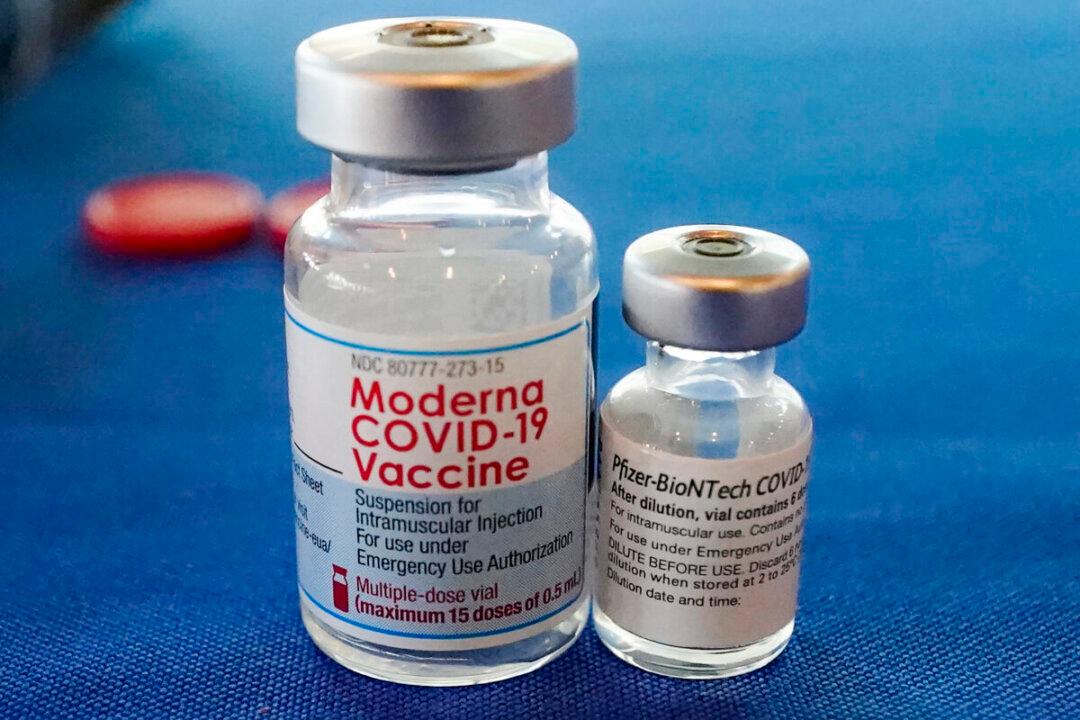
The idea of mixing and matching different COVID-19 vaccines is gaining momentum, with a special focus on letting Johnson & Johnson vaccine recipients get a dose produced by a different company.
The J&J shot produces inferior results compared to the vaccines that were created using messenger RNA technology, according to studies and real-world evidence. The main reason to allow heterologous vaccination schedules would be the aim of giving people who got a J&J vaccine to get a boost in protection.