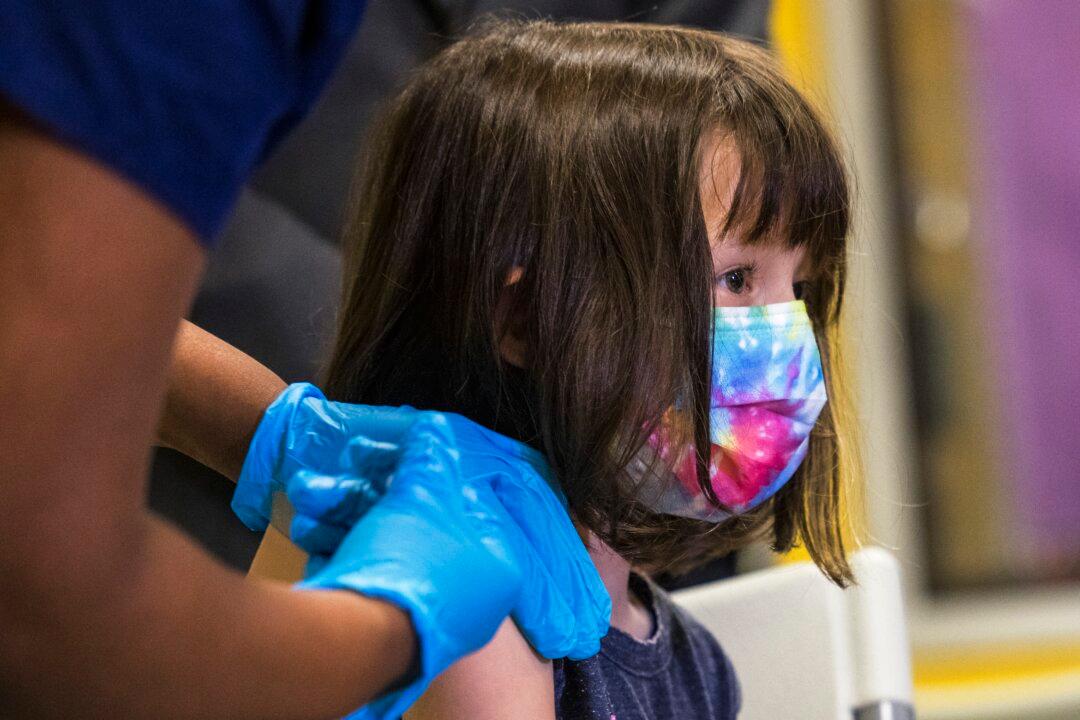
A Food and Drug Administration (FDA) advisory panel on June 15 voted to recommend the FDA give emergency authorization to the Moderna and Pfizer COVID-19 vaccines for children as young as 6 months of age.
The panel unanimously voted during a virtual meeting after presentations from the companies and the FDA.