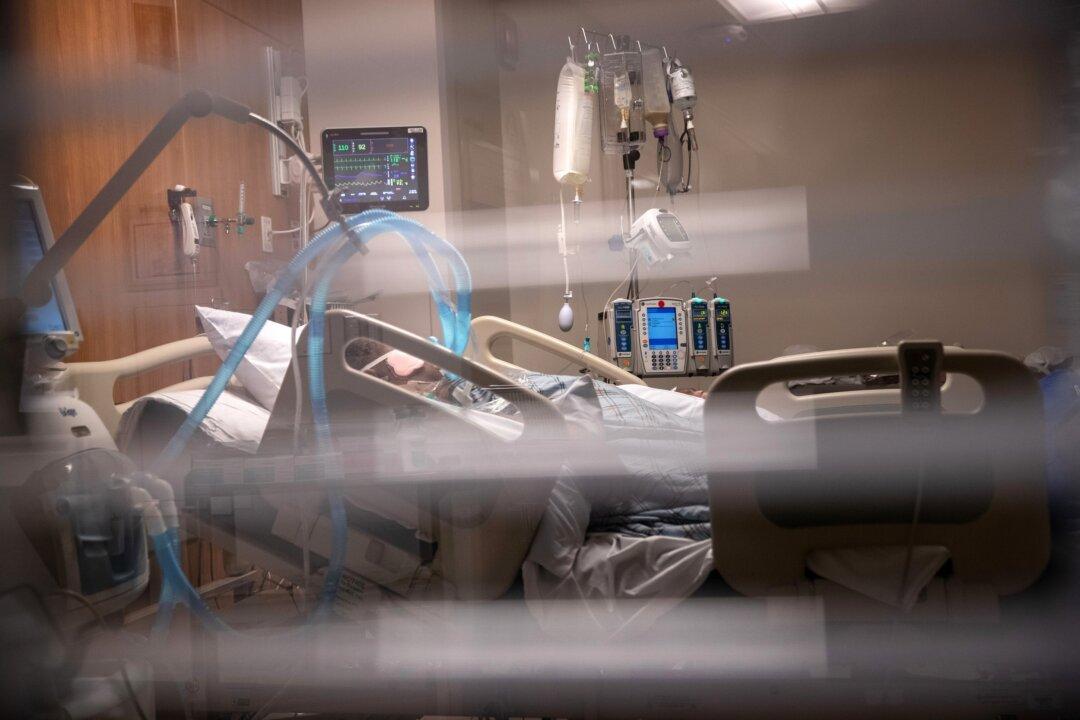
Two drugmakers plan on testing a drug that blocks enzymes in COVID-19 patients on ventilators in the United States.
The phase-three trial of Jakafi will start soon on patients who are on mechanical ventilation and who have acute respiratory distress syndrome, a type of respiratory failure characterized by rapid onset of widespread inflammation in the lungs, Novartis and Incyte announced Tuesday.