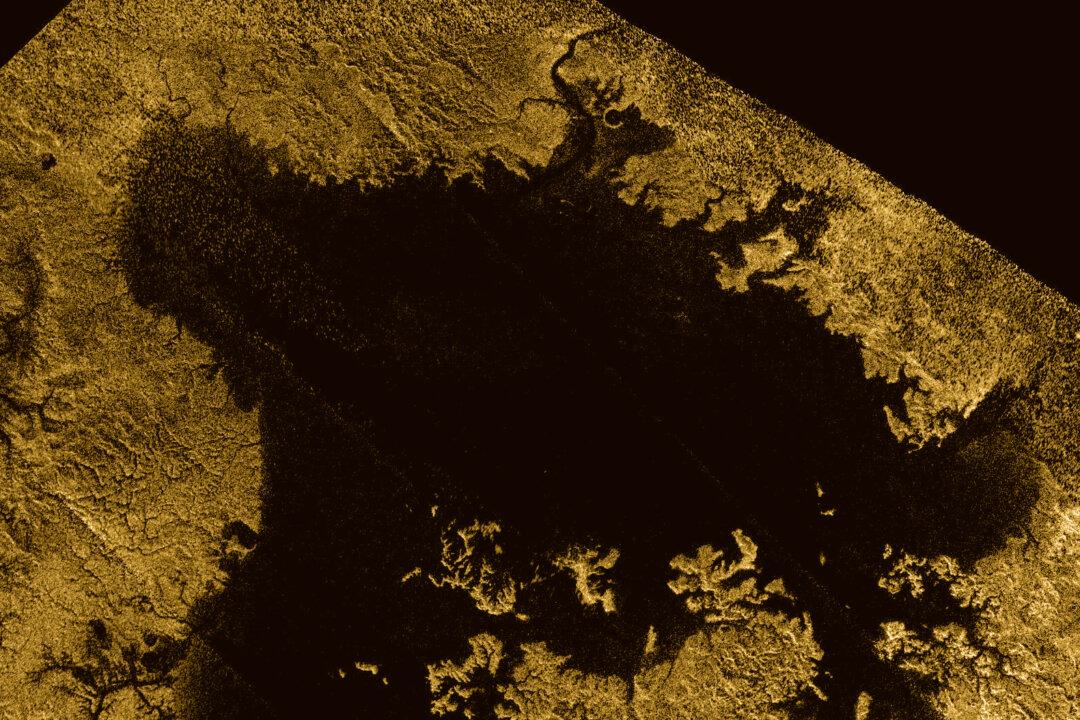
It’s not every day that you get to discover something new. But when you do it is a rather strange and quite brilliant feeling. You don’t really cry out “Eureka” (there’s usually about a million things going about it your head pointing out how it could be wrong). When you finally conquer the “wrong” demon and satisfy yourself that you have something new, well then you usually sit back in your chair and smile to yourself. Maybe at a push grab a cup of coffee and a celebratory chocolate bar from the vending machine. That’s pretty much how I felt when I worked out the latest crystal structure I’ve just published, of the “bath scum” of Titan.
The great thing about this column space is that I can use it to tell you all the back-story behind a paper, how it came about and why I think it’s really exciting.
Titan, the largest moon of Saturn, is in many ways pretty similar to Earth. It’s the only moon in the solar system with a substantial atmosphere, much thicker than our own. If you don’t mind the cold and lack of oxygen, moving about the surface there will feel a bit like walking under water. It’s pretty nice when you consider that the atmosphere most other planets and moons will barely shield you from the vacuum of space.